Can You Have West Nile Virus and Not Know It

American Crows (Corvus brachyrhynchos), some of the about susceptible birds to West Nile Virus infection. They are the most typically used bird species for WNV studies, and are utilized for national surveillance systems for avian deaths and WNV spread. Photo by Ingrid Taylar [four].
West Nile Virus, of the family Flaviviridae, is a zoonotic affliction that infects many species, including humans, throughout the world. Birds are the virus'southward primary natural reservoir, where they act as an amplifier host. However, their host competency (their ability to perpetuate the virus' development and spread) and response to infection varies betwixt species; they can have diverging viral loads, viremia, viral shedding, clinical signs, and morbidity. Many factors may impact a item organism and/or species' susceptibility and response to infection, including their immune-response capabilities, previous immunities, breeding systems, genetics, geographic spread, and stress levels. Overall, birds vary widely in how they are afflicted by West Nile Virus, and further surveillance and enquiry into WNV infections in birds is necessary to develop additional cognition and maintain preparedness for future outbreaks.
Initial Infection
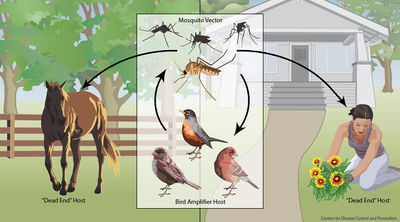
Due west Nile Virus transmission cycle. From the Centers for Affliction Control Website [21].
Birds may become infected with Westward Nile Virus in multiple means. The most common occurrence is through a mosquito vector [21]. Even so, studies have shown that they may contract the infection through ingestion of infected organisms or direct contact with infected materials.
Insect Vectors
Over forty species of mosquitoes have been institute to carry West Nile Virus. The most of import for spread is the genus Culex, which mainly feed on birds [i], [21]. If a mosquito feeds off an infected host with high plenty viral load (approximately ane meg virus particles per milliliter of claret), it will take up the virus into its gut. The virus will then multiply and spread to the rest of the mosquito's body, including its salivary glands [1]. The vector may and so feed off some other beast and transmit the virus to a new host.
Other blood sucking insects, such as ticks, have been found to be able to transmit the virus, though they are not as important for overall spread and outbreaks [one].
Oral Transmission
Birds may become infected past W Nile Virus through consuming infected prey items such every bit insects, small mammals, and other birds. After ingesting infected organisms, viremia usually occurs identically to that of mosquito-borne transmission [5]. This may influence incidence of infection in carnivorous birds, such as raptors and insectivores.
Contact Transmission
Enquiry has found that information technology is possible for birds to transmit the virus to one another every bit a consequence of emitting particles in oral or cloacal secretions [half dozen]. These secretions, such as feces, may contaminate h2o and food, or may directly contact another susceptible organism. In captivity this process is rare, and information technology is unknown how common information technology occurs in nature. It is hypothesized that this contact transmission road is a factor in spread of the virus in communal roosting populations and during breeding seasons [two], [6].
Species Susceptibility and Competence
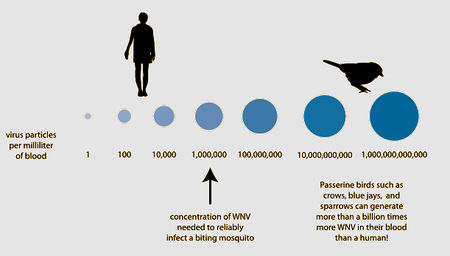
Chance of a mosquito picking upwardly West Nile Virus from an infected host. Birds are the primary competent host for viral spread through mosquitoes due to their loftier viral loads. Graphic from FAQ: West Nile Virus, July 2013. American Society For Microbiology [two].
| Tabular array of West Nile Virus host competency of 23 species of birds. A larger index number correlates to college amounts of viral load in concurrence with long durations of viremia. Data adjusted from Komar et al. 2003 [2]. | |
| Species | Reservoir Competence Index |
|---|---|
| Blueish Jay | 2.55 |
| Common Grackle | two.04 |
| House Finch | ane.76 |
| American Crow | 1.62 |
| Business firm Sparrow | ane.59 |
| Ring-billed Gull | 1.26 |
| Blackness-billed Magpie | one.08 |
| American Robin | one.08 |
| Ruddy-winged Blackbird | 0.99 |
| American Kestrel | 0.93 |
| Great Horned Owl | 0.88 |
| Killdeer | 0.87 |
| Fish Crow | 0.73 |
| Mallard | 0.48 |
| European Starling | 0.22 |
| Mourning Dove | 0.nineteen |
| Northern Flicker | 0.06 |
| Canada Goose | 0.03 |
| Rock Dove | 0 |
| American Coot | 0 |
| Ring-necked Pheasant | 0 |
| Monk Parakeet | 0 |
Most mammals, including horses and humans, are "dead-end" hosts for West Nile Virus, as, after infection, the virus does non highly concentrate enough in their claret to then be spread to insect vectors. Many bird species, withal, are important "amplification-hosts" for the spread of West Nile Virus, every bit the virus multiplies to exorbitant numbers within them. Some species can generate the highest levels of virus particles in their blood presently known for any viral infection; they tin can have millions of times more viral particles in their claret than similarly infected mammals [i].
Over 300 species of birds have been found infected with West Nile Virus [i]. Passerine birds (of the gild Passeriformes, such as flycatchers, robins, wrens, mockingbirds, and finches), charadiiform birds (of the social club Charadriiformes, such equally gulls, plovers, and stilts), and many raptors accept been plant to be competent hosts for viral infection and spread. In particular, Corvids (species of the family Corviade, including crows, ravens, magpies, and jays), as well as a few other sensitive species, such as American Kestrels and Slap-up Horned Owls, are competent and highly susceptible. Birds of the orders Anseriformes (ducks, geese), Columbiformes (pigeons), Galliformes (chickens, turkeys), Gruiformes (runway, coots), Piciformes (woodpeckers, toucans), and Psittaciformes (parrots) are generally less competent [2]. The competency levels of selected species of experimentally infected birds are shown in the tabular array to the left. These numbers were calculated using an equation that accounted for susceptibility (the proportion of birds that actually became infected after being experimentally exposed to the virus through oral or vector transmission), the bird's average daily infectiousness (the proportion of exposed vector mosquitoes that became infectious per day when feeding off these experimental birds), and duration of infectiousness (the number of days that the bird had high enough viral load to keep spread, determined from analyzing blood samples) [2]. Thus, birds with greater index numbers testify college amounts of viral load also as long durations of viremia, and are therefore better hosts for spreading infection. Species with depression index numbers are relatively unaffected or resistant to the virus.
Occurrence of bloodshed due to infection increases with species competence. In early on studies of WNV in the United States, up to 100% mortality was observed in American Crows (Corvus brachyrhynchos) and Blueish Jays (Cyanocitta cristata) experimentally inoculated with the virus. In these susceptible species, large amounts of virus are widely distributed in major organs, causing multi-organ failure and inducing a rapid expiry that does not let the development of clinical signs [6]. It is suspected that WNV infection may require underlying illnesses or immune suppression in item species to result in death [2]. Many symptoms, such equally neurological bug that impede flight and coordination, may lead to subsequent issues in survival that increase mortality in the wild [5].
It has been found that some species, particularly Corvids such every bit American Crows, may contain a high plenty viremia level that they remain reservoir competent postmortem; they are able to transmit viral particles to vector hosts that feed on their remains upwardly to 5 days after expiry [1], [7].
Many birds that contract W Nile Virus do not dice. Certain immune responses, such as antiviral mechanisms, may limit viral infection and viremic spread. Birds with depression susceptibility may present minor symptoms and recover, or may appear healthy with depression-grade, non-progressive infection. These birds may then have successful immune-mediated clearance of infectious particles [v]. Some species may also maintain low levels of viral replication that pb to chronic infections [8].
Infection Symptoms

An owl exhibiting symptoms of West Nile Virus infection: lethargy, head droop, and irregular body postition. Image from DNR - Due west Nile Virus (WNV). Michigan Section of Natural Resource [23].
The majority of individual birds infected with West Nile Virus practise not get ill or show symptoms. Nonetheless, if they practice become ill, clinical signs may include uncoordinated walking, lethargy, ataxia, weakness, tremors, inability to fly, anorexia followed by rapid weight loss, green waste products, blindness, lack of awareness, head droop, and abnormal body posture [3], [8], [23]. They may dice within 24 to 48 hours of showing symptoms [3]. In the terminal phase of infection preceding death, birds may have astringent tremors or seizures. These symptoms reflect virus infection and inflammation of the brain and spinal cord [3], [8].
Through bird necropsies, organs found to be well-nigh oft infected with virus particles are the spleen, kidney, skin, and eye [5]. Unremarkably, the virus first appears in the spleen, and so quickly spreads to other organs, and reaches the central nervous system afterward. Tissue distribution occurs earlier in more susceptible species. Histologic lesions, such as mild to severe encephalitis, necrosis, inflammation, and hemorrhages, have been found in multiple tissues, including the brain, spinal cord, heart, peripheral nervous system, heart, spleen, liver, kidney, lung, gastrointestinal tract, endocrine arrangement, gonads, skeletal muscle, peel, and bone marrow. The speed of viral distribution and host death may affect the evolution of lesions, as hosts with higher susceptibility may die before lesions get observable. Macroscopic changes due to infection include emaciation, aridity, multi-organ hemorrhages, and congestion [5], [8].
Issues with movement and flight in wild birds, equally opposed to internal issues, leads more than frequently to human notice, interaction, and intervention. Thus, neurological symptoms are very normally identified symptoms of the virus. And although they are very frequently infected by WNV, Corvids rarely nowadays neurological symptoms, as the virus spreads so quickly that the bird dies before presenting most issues; many dice from lesions of their internal organs before the viral particles reach the brain or spinal string [eight]. Less susceptible birds, such every bit owls and hawks, oftentimes live long plenty to testify brain and spinal string lesions, and thus more ordinarily nowadays neurological issues [5].
Host Factors
There is fiddling evidence about why bird species are affected by W Nile Virus differently. Some speculation and written report points to life-fashion variations, such as breeding systems and geographic spread, which pb to unique collections of pathogen defense components for each species. Other differences may arise from genetics, such as intrinsic capabilities to combat viral infection.
Geography
Phylogenetically related, geographically divergent species have been shown to be differently affected past Due west Nile Virus infection [10]. Thus, their geographic spread is thought to influence their susceptibility. It is believed that this is through climate differences influencing the acquisition of cantankerous-reactive immunologic protection to Westward Nile Virus. Sometimes, when a bird is infected with 1 kind of virus, it will gain immune protection against other related viruses. Warmer habitats support greater vector populations likewise as meliorate conditions for viral propagation, so southern-living species, where the climate is warmer, are more frequently exposed to mosquito built-in Flaviviridae infections, and therefore may develop better resistance to WNV [10].
There is some speculation that differences in species susceptibility may be related to co-evolution of the virus with item species. In the Old-Globe (Europe, Africa, Asia), West Nile virus is generally a benign infection in birds, maybe due to thousands of years of evolutionary selection for birds that produce antibodies and therefore survive. By and large, Old-World birds (such as Barn Owls, Tyto alba, native to Europe) appear to exist less susceptible to WNV than New-world birds (such equally Cherry-tailed Hawks, Buteo jamaicensis and Great Horned Owls, Bubo virginianus, native to the Americas) [5]. It wasn't until 1999 that the virus first appeared in North America. Therefore, it may be that because Erstwhile-Earth birds have been in contact with the virus for longer, they take evolved better immune responses to it [11], [12].
Convenance Systems
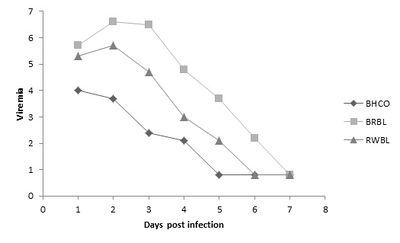
The viremia response of taxonomically similar bird species to West Nile Virus infection. BHCO=Brownish-Headed Cowbird (Molothrus ater), BRBL=Brewer'southward Blackbird (Euphagus cyanocephalus), RWBL=Red-winged Blackbird (Agelaius phoeniceus). Lower viremia of BHCO may exist explained by their parasitic breeding system. Data adapted from Reisen, West.K., and D.C. Hahn. 2007 [thirteen].
Taxonomically similar species may diverge in viral susceptibility due to their type of breeding organisation. In particular, it has been found that parasitic breeding may increase resistance to WNV, as they present lower viremia levels [xiii]. Breed parasite bird species, such as Brown-Headed Cowbirds (Molothrus ater), may develop a robust allowed system and innate affliction response. As shown in the figure to the right, the Dark-brown-headed Cowbird, every bit compared to related species, consistently presents the lowest and fastest decreasing viremia when experimentally infected with WNV by mosquito vectors [13]. This is because they are in close contact with varying host bird species, and thus experience infestation with a greater diversity of vector species than do other songbirds [13]. Therefore, this life-fashion evidently influences their susceptibility to WNV.
Considering information technology is possible for infection to spread through direct contact between birds, species that that accept collective breeding patterns, or those that have grouping roosting, feeding, or migration stopovers sites, as well as other similar behaviors that increment grouping densities, may be more than prone to spreading infection to one some other [2], [24]. Spread may also increment through mosquito vectors because of the proximity and availability of hosts in these situations. These factors may lead to an increase in notable effect on these kinds of species' populations.
Genetics
Few studies look at the genetic footing for species' host capabilities for WNV. However, at that place has recently been research into a potential genetic basis of WNV resistance in item bird hosts. One gene, two'-v' oligoadenylate synthetase 1b (Oas1b), codes for an enzyme that was previously constitute in mice to play a part in WNV resistance, and has been found in chickens to show similar oligoadenylate synthetase activity [14]. This chicken OAS-L enzyme, when expressed in mammalian cells, has antiviral activity against WNV. Chickens, compared to many other bird species, are relatively resistant to WNV infection. This gene may be responsible for their particular immune abilities to fight the virus [xiv].
Because Corvids, a related group of birds, are collectively more susceptible to infection, it is clear that they have a similar reason for increased detrimental effect. Their allowed systems react slowly to initial infection, which may exist caused past genetic factors. This slow allowed reaction allows the virus to spread through their organs quickly and cause severe harm. This is in opposition to birds with lower susceptibility, which show lower speed of viremia spread, and thus accept a improve chance of fighting viral load increase or harm to vital organs.
Effect on Populations
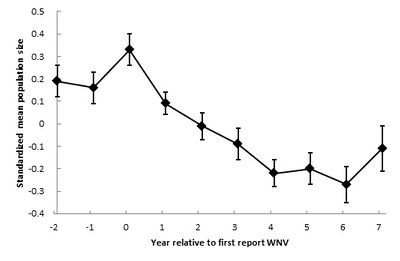
Crow population changes (± SE) after West Nile virus was first reported in the United states. Data adjusted from Koenig et al. 2010 [15].
Individual animals vary in their responses to WNV infection; in the wild, birds are frequently found with WNV antibody prevalence, suggesting previous WNV infection and subsequent survival. American Crows, which experimentally prove a very high mortality rate (upwards of 100%), take been institute with antibodies to the virus [17]. Even so, the virus generally furnishings species in trends of greater or lesser affect, as discussed above. Thus, effects due to WNV to species total and regional populations have been studied since the virus was kickoff detected in the United States in 1999.
Crows and "Dilution Upshot"
Inside 4 months of West Nile Virus's initial introduction, approximately five,500 crows died of infection. Past 2001, the virus spread to bird populations in many eastern states. Past 2002 information technology spread to most of the mid-due west likewise equally California, and by 2004 had been detected in every state of the continental United states, excluding Oregon [15].
Crow populations have been the nearly looked at and analyzed for how they have been effected by the viral outbreak, and changes to their populations have been studied over time. One analysis of American Crow populations describes that initially populations declined rapidly, and so lessened in refuse, and are soon normalizing, every bit displayed in the effigy to the left. Thus, West Nile virus seemingly became less virulent as it moved westward beyond Northward America [15]. The researchers discuss that the virus potentially evolved as information technology spread. As an ssRNA virus, West Nile presents a big amount of genetic variation. The viral strains that killed their hosts too quickly could not be effectively spread through mosquito vectors, nor moved regionally through migratory flight patterns, and therefore the only strains that were able to exist spread due west were those to be lower in lethality. This characteristic of a lower level of lethality of the virus may explain the lessening of population decline observed today [16].
These researchers also establish that crows in diverse habitats were less likely to become infected with the affliction than crows in species-poor areas. They explain the decrease in infection and population turn down through the thought of a "dilution effect." Areas of higher diversity in bird populations were less afflicted by the virus, because it was less likely for a vector mosquito to bite a very susceptible bird; they were more likely to infect "dead-end" host species [fifteen], [16].
Some studies which take compared the furnishings of West Nile Virus on different species have plant evidence for possible changes in species' ranges, potentially due to deaths of some other species' population caused by WNV infection. Fish Crows, which testify lower mortality rates to WNV than do American Crows, are usually plant in small regions near coasts. Researchers constitute that some populations seem to have begun growing their ranges, moving inland [17]. They hypothesize that the increase in Fish Crow range may be a issue of the decrease in American Crow populations from WNV mortality. The Fish Crows may be taking the open niches that American Crow populations have left as their numbers have decreased. More research should be pursued into this surface area, as it may represent ecological furnishings and disturbances [17].
2012 Hawkeye Deaths
In belatedly 2012, over 2 dozen Bald Eagles (Haliaeetus leucocephalus) succumbed to W Nile Virus infection in Utah [eighteen]. Their deaths caused lots of press coverage because of the irregularity of their deaths, also as their position every bit a symbol of the country. The birds were beingness plant laying listless on the footing, many suffering from head tremors, seizures, and paralysis in the legs, anxiety, and wings. Because this occurred in the wintertime, it is suspected that mosquitoes were non responsible for the virus's spread. Researchers believe that the eagles may have contracted WNV from eating dead grebes. Grebes are prevalent in large numbers in the area in the winter months, and may take been affected by an outbreak of WNV. The deaths of these Bald Eagles will well-nigh likely not affect the greater population of Bald Eagles in the Us [18], [25].
Endangered Species
There is business organization that endangered species may exist at risk of further population damage from a WNV outbreak. Such threatened species include the Florida Scrub-Jay (Aphelocoma coerulescens), California Condor (Gymnogyps californianus), and Whooping Crane (Grus americana) [12]. Since 1999, no dramatic changes to endangered bird species has been shown to be a upshot of WNV infection. Still, considering the virus is nonetheless prevalent across the country, there is a standing possibility of future outbreaks [1], [12]. If changes do occur, organizations are in place to observe them and answer accordingly; currently, surveillance on endangered species populations is largely in place, and tracking of WNV spread is in progress as well (see below department "Time to come Studies and Surveillance").
Surveillance and the Future of Infection
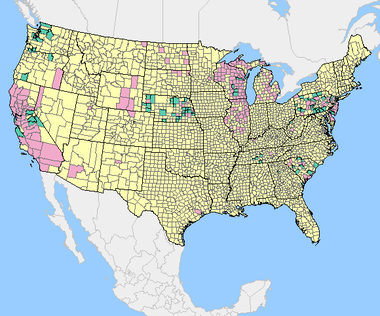
USGS cumulative 2013 data of WNV in sentinel birds equally of January 07, 2014. Pink=positive exam results, green=samples submitted, yellow=no postitive tests (either because of no surveillance performed or no positive results) [19].
Fifty-fifty though the effects and spread of W Nile virus take recently declined/stagnated [15], it is necessity to continue research and surveillance of this disease. Some support includes the fact that the affliction is all the same frequently detected and that it has been identified at least one time in every state [ane]. Furthermore, the evidence that West Nile Virus had adaptively evolved to lower lethality to encourage spread [15] illustrates its potential for recurrence and more dangerous outbreaks. Another modify in viral strain may occur, as it is clear that the genetic variation of the virus allows it to take adaptable abilities.
To farther study the spread and event of WNV, surveillance systems have been put into place by the CDC and USGS (United States Geological Survey) [19]. Many states, including California, Pennsylvania, Illinois, Ohio, and many others encourage their residents to report dead or sick birds to governmental services; each state has a website easily establish online where residents can contact and provide information to officials. The USGS tracks and reports WNV in U.s.a. counties by obtaining data regarding arboviral cases through an agreement with the Centers for Disease Control and Prevention (CDC), and this data is used to gain further knowledge about disease spread. They by and large use picket chickens for their enquiry [xix]. The map of the U.s. to the right is a recently produced and oftentimes modified figure available online to give information to the public about where WNV is currently tested for and being plant.
Currently, a commercial West Nile Virus vaccine exists for horses [9], while no vaccine for humans or birds exists for common employ [21]. Inquiry has been going into developing bird vaccines, and some advancements have been established. Several zoos provide vaccinations to their birds with intramuscular commercially available inactivated whole virus vaccine, and have institute meaning differences in antibody titers in particular species, including American flamingos (Phoenicopterus ruber) and black-footed penguins (Spheniscus demersus) [20]. Further studies accept been done to exam the use of other types of WNV vaccines in varying bird species. One research group injected DNA vaccinations against WNV into Red-Tailed Hawks (Buteo jamaicensis) and found that vaccinated birds presented seroconversion. They had less astringent viremia with shorter, lower-intensity shedding periods than did non-vaccinated animals. The vaccine did not completely eliminate affliction, but successfully weakened the effects of infection [22]. More enquiry is warranted to discover better and stronger vaccines against WNV for birds.
In caring for sick animals found past the public, wildlife rehabilitation centers frequently come up beyond birds with West Nile Virus infections. These organizations keep rail of birds they deem as potentially West Nile positive. All the same, testing to confirm infection is expensive. Unremarkably, decision of disease is concluded based on symptoms; sometimes oral samples are collected, simply only later on the presentation of symptoms or expiry [23]. Thus, many not-symptomatic cases may be left unnoticed. Numerous birds that die in the wild may also go undetected. Thus, further academic research and testing of birds is necessary to go along advancing knowledge about the virus and developing preparedness confronting outbreaks.
Further Reading
West Nile Virus— Centers for Disease Control and Prevention
Due west Nile Virus— Wikipedia
References
one. Greene, S. E., and A. Reid. 2013. FAQ: Westward Nile Virus. American Society For Microbiology.
2. Komar, North., S. Langevin, S. Hinten, N. Nemeth, E. Edwards, D. Hettler, B. Davis, R. Bowen, and M. Bunning. 2003. Experimental Infection of North American Birds with the New York 1999 Strain of Due west Nile Virus. Emerging Infectious Diseases 9(3): 311-322.
three. "West Nile Virus." Seattle Audubon Society. Spider web. ix Mar. 2014. <http://www.seattleaudubon.org/sas/LearnAboutBirds/SeasonalFacts/WestNileVirus.aspx>.
iv. Taylar, I. Crows. 2012. Seattle, WA. flickr. Web. ix Mar. 2014. <http://www.flickr.com/photos/taylar/8516674909/>.
5. Nemeth, Due north., D. Gould, R. Bowen, and N. Komar. 2006. Natural And Experimental Due west Nile Virus Infection In Five Raptor Species. Periodical of Wildlife Diseases 42(1): 1-xiii.
6. Kipp A.M., J.A. Lehman, R.A. Bowen, P.East. Pull a fast one on, M.R. Stephens, Thou. Klenk, Northward. Komar, and Thou.L. Bunning. 2006. West Nile virus quantification in carrion of experimentally infected American and fish crows. American Periodical of Tropical Medicine and Hygine 75(iv): 688-690.
7. McLean, R.Grand. 2004. West Nile Virus: Impact on Crow Populations in the United States. USDA National Wild fauna Inquiry Center - Staff Publications 367. nine Mar. 2014. <http://digitalcommons.unl.edu/icwdm_usdanwrc/367>.
eight. Gamino, V., and U. Höfle. 2013. Pathology and tissue tropism of natural West Nile virus infection in birds: a review. Veterinary Research 44(1): 39.
9. Frequently Asked Questions virtually West Nile Virus and Wildlife. USGS National Wildlife Health Center. Web. 9 Mar. 2014. <http://world wide web.nwhc.usgs.gov/disease_information/west_nile_virus/frequently_asked_questions.jsp>.
10. Lopes, H., P. Redig, A. Glaser, A. Armien, and A. Wünschmann. 2007. Clinical Findings, Lesions, and Viral Antigen Distribution in Great Greyness Owls (Strix nebulosa) and Barred Owls (Strix varia) with Spontaneous West Nile Virus Infection. Avian Diseases 51(1): 140-145.
eleven. Ludwig, G.V., P.P. Calle, J.A. Mangiafico, B.L. Raphael, D.K Danner, J.A. Hile, T.Fifty. Clippinger, J.F. Smith, R.A. Melt, T. McNamara. 2002. An outbreak of West Nile virus in a New York City captive wild fauna population. American Journal of Tropical Medicine and Hygine 67(1): 67-75.
12. Chu, M., Due west. Stone, K.J. McGowan, A.A. Dhondt, W.M. Hochachka, and J.E. Therrien. 2003. West Nile File. Cornell Lab of Ornithology, Birdscope 17(ane). Spider web. eleven Mar. 2014. <http://www.birds.cornell.edu/Publications/Birdscope/Winter2003/West_Nile_File.html>.
thirteen. Reisen, W.K., and D.C. Hahn. 2007. Comparison of Immune Responses of Brownish-Headed Cowbird and Related Blackbirds to West Nile and Other Mosquito-Borne Encephalitis Viruses. Periodical of Wild fauna Diseases 43(3): 439-449.
xiv. Tag-El-Din-Hassan H.T., N. Sasaki, Grand. Moritoh, D. Torigoe, A. Maeda, and T. Agui. 2012. The chicken 2'-5' oligoadenylate synthetase A inhibits replication of West Nile Virus. Japan Journal of Veterinary Inquiry 60(2-3): 95-103.
15. Koenig, West.D., Westward.M. Hochachka, B. Zuckerberg, and J.Fifty. Dickinson. 2010. Ecological determinants of American crow bloodshed due to Due west Nile virus during its North American sweep. Oecologia 163(4): 903-909.
16. Powell, H. 2014. All Well-nigh Birds - Counting Crows - Due west Nile virus effect on American Crows. Cornell Lab of Ornithology, Web. 13 Mar. 2014. <http://www.allaboutbirds.org/Folio.aspx?pid=1956>.
17. Wilcox, B.R., Thousand.J. Yabsley, A.E. Ellis, D.E. Stallknecht, and S.East.J. Gibbs. 2007. Due west Nile Virus Antibiotic Prevalence in American Crows (Corvus brachyrhynchos) and Fish Crows (Corvus ossifragus) in Georgia, U.South.A. Avian Diseases 51(ane): 125-128.
eighteen. Glionna, J.M. 2014. West Nile virus blamed for rash of bald eagle deaths in Utah. Los Angeles Times, 3 January. sec. Nation Now. Print.
nineteen. Due west Nile Virus Maps - Bird - U.s.a.. United States Geological Survey. U.South. Section of the Interior. Web. 11 Mar. 2014. <http://diseasemaps.usgs.gov/wnv_us_bird.html>.
xx. Okeson, D.Thou., S.Y. Llizo, C.L. Miller, and A.50. Glaser. 2007. Antibiotic Response Of Five Bird Species Later Vaccination With A Killed West Nile Virus Vaccine. Journal of Zoo and Wild fauna Medicine 38(two): 240-244.
21. West Nile Virus. 2014. Centers for Disease Control and Prevention. Spider web. 24 Mar. 2014. <http://world wide web.cdc.gov/westnile/alphabetize.html>.
22. Redig P.T., T.North. Tully, B.Westward. Ritchie, A.F. Roy, M.A. Baudena, and 1000.J. Chang. 2011. Effect of West Nile virus DNA-plasmid vaccination on response to live virus challenge in reddish-tailed hawks (Buteo jamaicensis). American Journal of Veterinary Research 72(eight): 1065-1070.
23. DNR - Due west Nile Virus (WNV). Michigan Department of Natural Resources. Web. ten Mar. 2014. <http://www.michigan.gov/dnr/0,4570,7-153-10370_12150_12220-99070--,00.html>.
24. Pérez-Ramírez, E., M.A. Jiménez-Clavero, and F. Llorente. 2014. Experimental Infections of Wild Birds with West Nile Virus. Viruses 13(2): 752-781.
25. Soaring deaths of bald eagles in Utah attributed to West Nile virus. 2013. NBC News, December 31. Web. one Apr. 2014. <http://usnews.nbcnews.com/_news/2013/12/31/22125743-soaring-deaths-of-bald-eagles-in-utah-attributed-to-due west-nile-virus?calorie-free>.
Edited by Leah Pomerantz, a educatee of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2014.
Source: https://microbewiki.kenyon.edu/index.php/West_Nile_Virus_in_Birds
Post a Comment for "Can You Have West Nile Virus and Not Know It"